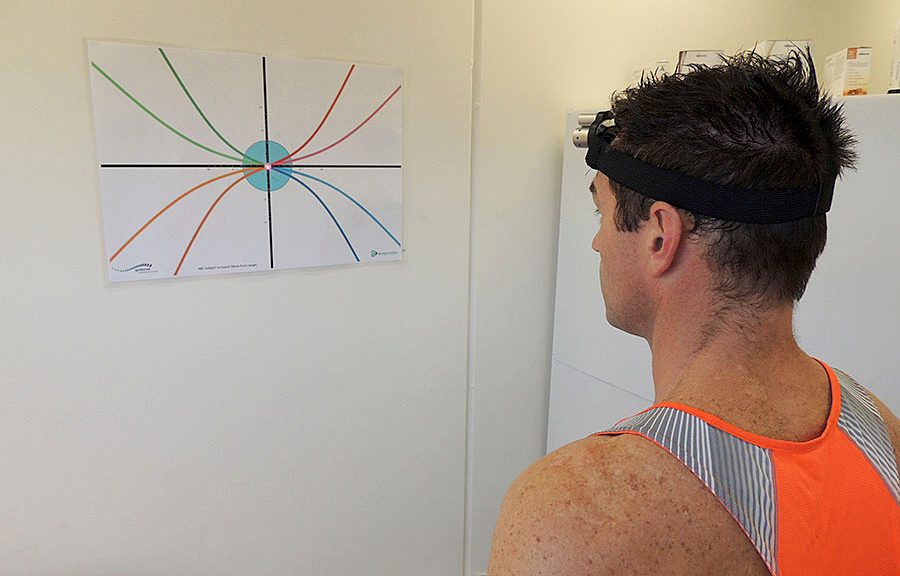
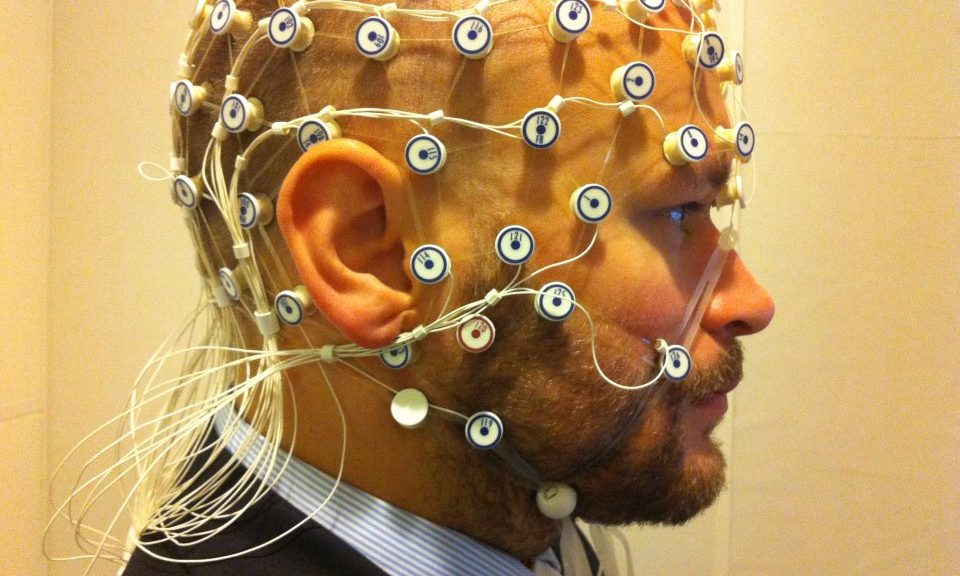
There is considerable evidence to support the importance of cervical afferent dysfunction in the development of dizziness, unsteadiness, visual disturbances, altered balance, and altered eye and head movement control following neck trauma, especially in those with persistent symptoms.(1) The evaluation of potential impairments (altered cervical joint position and movement sense, static and dynamic balance, and ocular mobility and coordination) should become an essential part of the routine assessment of those with traumatic neck pain, including those with concomitant injuries such as concussion and vestibular or visual pathology or deficits.(1)
Dizziness that occurs after concussion/mTBI presents with varied characteristics and several potential sources and mechanisms, including the inner ear, the brain, the cervical spine, and/or the integration of afferent input and tuning within the sensorimotor control system.(2) Vestibular rehabilitation therapy (VRT) has been used to treat persistent dizziness after concussion/mTBI, but recent attention has examined the possible role of the cervical spine in post-concussive dizziness.(2) Dizziness after concussion/mTBI has been shown to improve when manual therapy and specific sensorimotor control exercises for the cervical spine were added to standard care VRT.(2)
Hammerle et al (2) compared traditional VRT with cervical spine proprioceptive retraining (CSPR) in patients with recurring dizziness after concussion/mTBI who had at least 1 abnormal cervical spine proprioceptive test (e.g. cervical joint position error or smooth pursuit neck torsion test), regardless of the presence or absence of neck pain. Patients were excluded from the study if they had dizziness with a clear peripheral vestibular or central symptom origin (e.g. BPPV, consistent saccadic intrusions on smooth pursuits, gaze holding nystagmus, loss of gaze holding during VOR cancellation testing). The results demonstrated that patients who received CSPR were 30 times more likely to report improvement in dizziness symptoms compared with those who received VRT when abnormal CSP tests were present.(2)
References:
1. Treleaven J. Dizziness, Unsteadiness, Visual Disturbances, and Sensorimotor Control in Traumatic Neck Pain. J Orthop Sports Phys Ther [Internet]. 2017;47(7):492–502. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=28622488&retmode=ref&cmd=prlinks
2. Hammerle M, Swan AA, Nelson JT, Treleaven JM. Retrospective Review: Effectiveness of Cervical Proprioception Retraining for Dizziness After Mild Traumatic Brain Injury in a Military Population With Abnormal Cervical Proprioception. J Manipulative Physiol Ther [Internet]. 2019;42(6):399–406. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0161475418300411